What Causes a Molecule to Have a Net Dipole Moment
In H_2O molecule the oxygen atom being much more electronegative than the hydrogen atom will cause the. Only cis3-hexane is unsymmetrical molecule.
On What Factors Does The Dipole Moment Of Organic Compounds Depend On Quora
It has dipole moment.
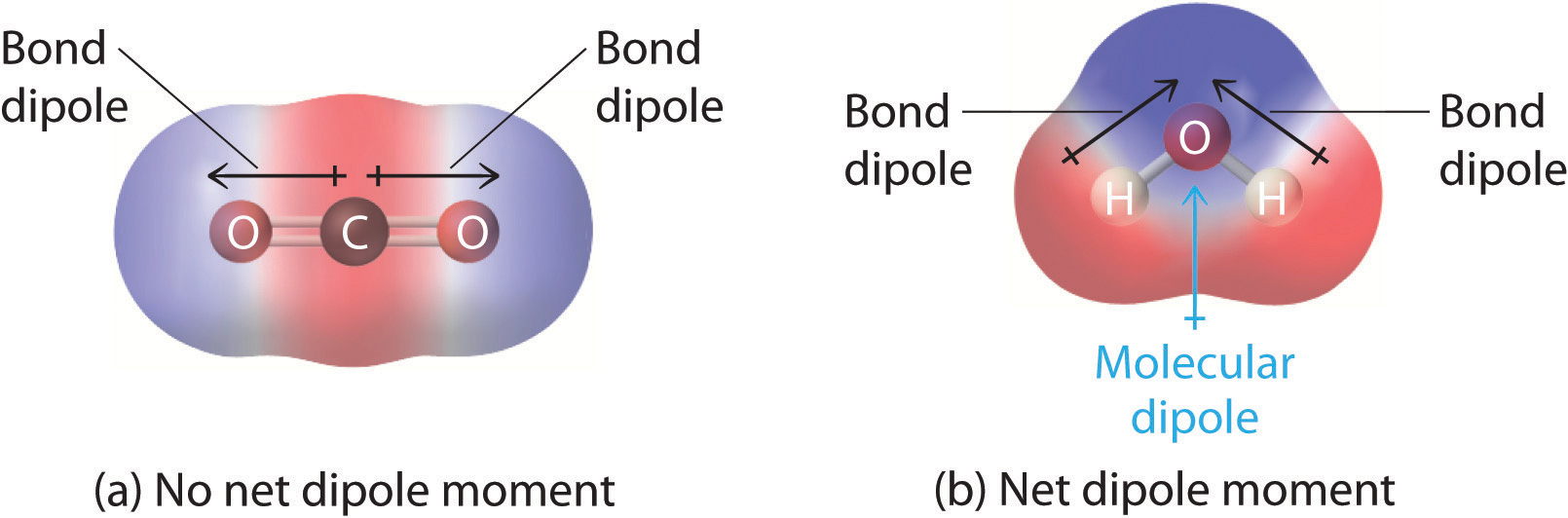
. The bond dipoles cannot cancel one another so the molecule has a net dipole moment. Dec 20 2017. Its asymmetrically placed carboxylic acid group and hydroxyl group cause it to have a net dipole moment which grants it its polarity.
Generally Polar compounds do not have zero dipole moment. Polar or nonpolar Q. The main cause for the development of the dipole moment is the electronegativity difference between chemically bonded atoms or elements.
HCl is a diatomic molecule with dissimilar atoms. As a molecule vibrates if there is a fluctuation in its dipole moment then this induces an electric field that interacts with the electric field associated with the infra red radiation. The compound which has same groups on both the carbons of CC will have less dipole moment than the compounds having different groups.
Does the molecule BF 2 Cl have a dipole moment. These are molecules with polar bonds caused by a diference in. Has a perfect tetrahedral geometry with all side atoms same.
An acetone molecule does have a net dipole. CHF_3 is a polar molecule. The separation of charges in any system leads to a dipole moment.
2 2 3 3 -Tetrammethyl butane. Compound I and III have same g view the full answer. Learn this topic by watching Molecular Polarity Concept Videos All Chemistry Practice Problems Molecular Polarity Practice Problems Q.
Both ionic and covalently bonded compounds develop dipole moments. Dipole moments occur due to atoms electronegativity where one atom has the ability to attract electrons towards it giving the electrons a negative and a positive charge. Having partial positive and partial negative charges from polar bonds arranged asymmetrically.
Draw the Lewis structures of CF4 and CF2CCl2a. The atoms are both neutral so the atom has no dipole moment. The presence of Van der Waals forces C.
This is caused by the difference inelectronegativity between methyl groups and a. Dipole moments occur due to the difference in electronegativity between two chemically bonded atoms. The total number of electrons around the central atom S is eight which gives four electron pairs.
Salicylic acid is also known as 2-hydroxybenzoic acid and 2-carboxyphenol. If there is a match in frequency of the radiation and the natural vibration of the molecule absorption occurs. Only unsymmetrical molecules have dipole moment.
See full answer below. Due to difference in electronegativities of the 2 atoms. The presence of a single polar bond causes the molecule to have a net dipole moment μ μ because the electron density.
This occurs due to an atoms electronegativity - where one atom has the ability to attract electrons towards it In other words electrons wants to spend other time around it giving it a negative charge and the other a positive charge. Electronegativity of B A. So it will have dipole dipole interaction along with the weaker dispersion forces.
This means that salicylic acid contains a benzene ring a hydroxyl group attached to one of the carbon atoms and a carboxylic acid group attached to. Has a perfect tetrahedral geometry but due to the presence of dissimilar groups its net dipole moment is not zero. In CH 2 F 2 the hydrogen is less electronegative than the carbon this means there will be a net movement of electrons towards the fluorine and the molecule will be polar and have a net dipole moment.
The presence of a net charge that does not cancel out D. A ll molecules will have London dispersion forces which get stronger as the molecule gets heavier more electrons causes a shift in electron cloud distribution resulting in a temporary dipole. What causes a molecule to have a net dipole moment.
The overall dipole moment of a molecule is obtained by vectorial addition of different bond moments which arise due to unsymmetrical distribution of bond pair electrons as shown below. Dipole moments occur when there is a separation of charge. In a molecule if there is a difference between the electronegativity of two atoms then there exist a polarity in the bond.
Solve any question of Chemical Bonding and Molecular Structure with-. They can therefore arise in ionic bonds as well as in covalent bonds. The dipole moment ofacetone is 291 D.
Is no net movement of electrons and a non-polar molecule with no net dipole moment. To find out which molecule has zero dipole moment we need the net dipole moment of the given compounds and for that we need to check the polarity of the compounds. A polar molecule has a net dipole as a result of the opposing charges ie.
Ay molecule with a net dipole moment will have dipole -dipole interactions. Click to see full answer. Due to symmetry this molecule has a net zero dipole moment.
In order for a molecule to have a net dipole moment it has to have an asymmetric separation of charges. What is the hybridization of the carbon atoms in these compoundsb. Water H2O is an example of a polar molecule since it has a slight positive charge on one side and a slight negative charge on the other.
Two of these electron pairs are bonding pairs and two are lone pairs so the molecular geometry of H 2 S is bent Figure 226. Correct option is B Dipole moment is vector quantity. The dipole moment of a molecule is the measure up of its polarity.
A bond dipole moment is a measure of the polarity of a chemical bond between two atoms in a molecule. Dipole moments occur when there is a separation of charge. A dipole moment arises in any system in which there is a separation of charge.
The polarity of a molecule is straight proportional to. In O2 theres no separation of charges. The polarity that a molecule is directly proportional come the distinction in the electronegativity the atoms.
The presence of only nonpolar bonds in the molecule B. 16 Suppose that a molecule has the formula AB 3. The presence of both covalent and ionic bonds in the molecule.
Why Is A Molecular Dipole Moment Zero In Symmetrical Molecules Quora

2 2 Polar Covalent Bonds Dipole Moments Chemistry Libretexts

Comments
Post a Comment